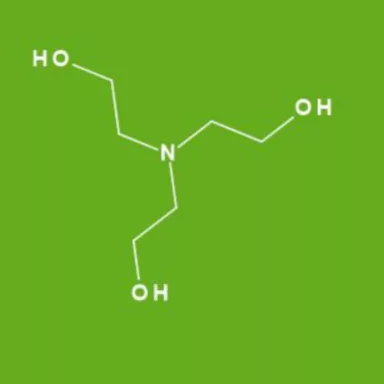
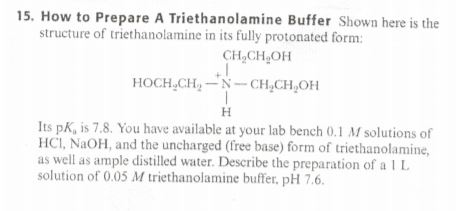
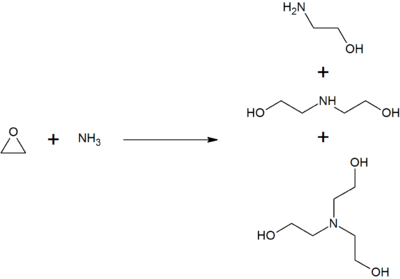Triethanolamine as an Efficient and Reusable Base, Ligand and Reaction Medium for Phosphane-Free Palladium-Catalyzed Heck Reactions

Triethanolamine-based protic ionic liquids with various sulfonic acids: Synthesis and properties - ScienceDirect

Unexpected Roles of Triethanolamine in the Photochemical Reduction of CO2 to Formate by Ruthenium Complexes | Journal of the American Chemical Society

Triethanolamine as an Efficient and Reusable Base, Ligand and Reaction Medium for Phosphane‐Free Palladium‐Catalyzed Heck Reactions - Li - 2006 - European Journal of Organic Chemistry - Wiley Online Library

Furosemide:Triethanolamine Salt as a Strategy To Improve the Biopharmaceutical Properties and Photostability of the Drug | Crystal Growth & Design

Synthesis and properties of triethanolamine-based salts with mineral and organic acids as protic ionic liquids - ScienceDirect

![T23040-4.0 - Triethanolamine, Free Base [2,2',2"-Nitrilothriethanol], 4 Liter T23040-4.0 - Triethanolamine, Free Base [2,2',2"-Nitrilothriethanol], 4 Liter](https://d2gdaxkudte5p.cloudfront.net/system/images/T23040-4.0_.jpg)
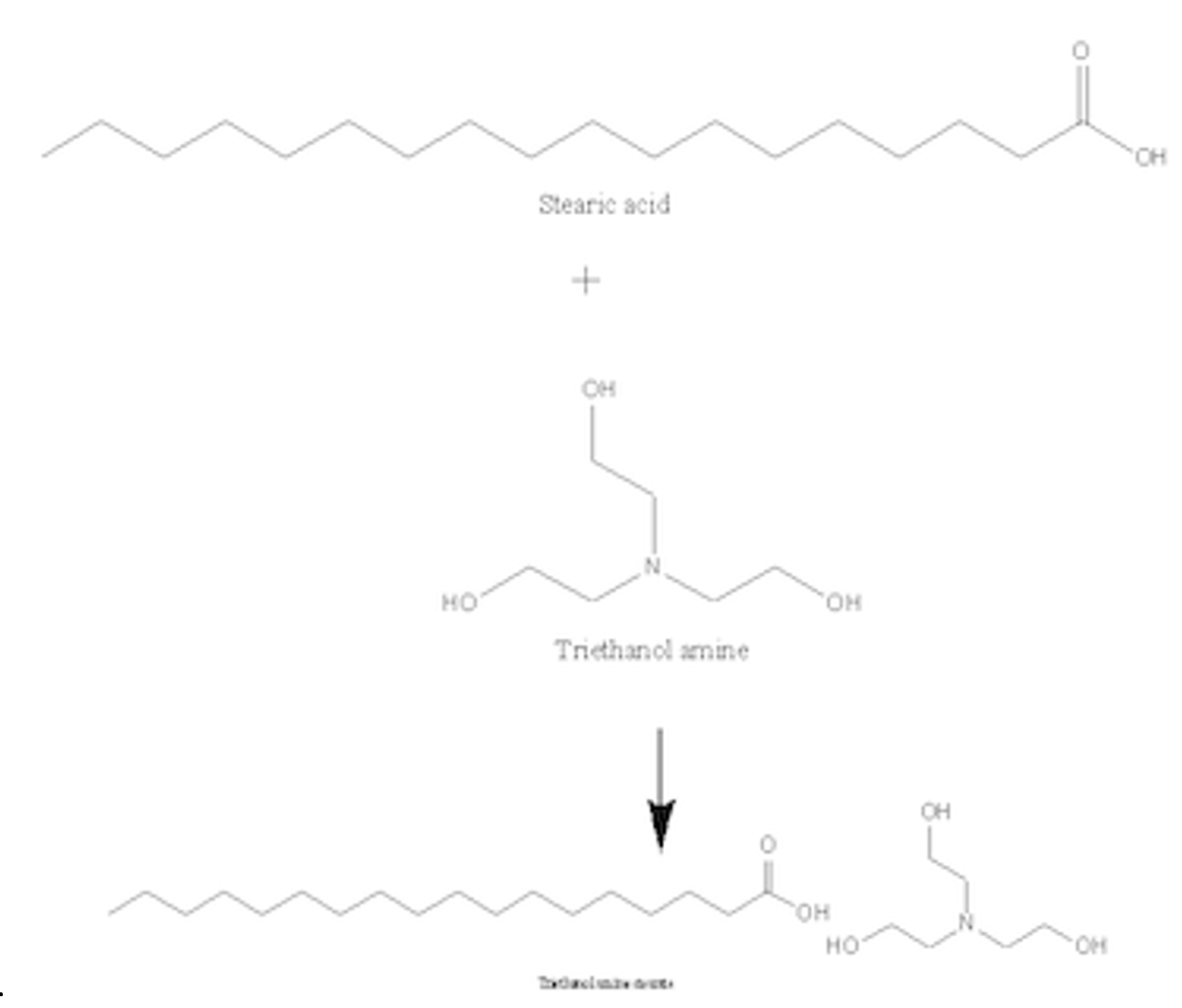
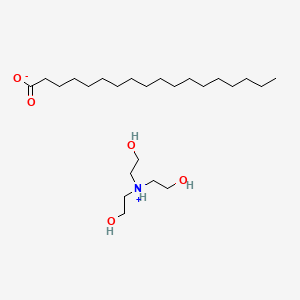
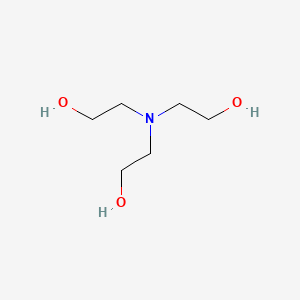

![T23040-1.0 - Triethanolamine, Free Base [2,2',2"-Nitrilothriethanol], 1 Liter T23040-1.0 - Triethanolamine, Free Base [2,2',2"-Nitrilothriethanol], 1 Liter](https://d2gdaxkudte5p.cloudfront.net/system/images/plabel_14881_20230721-085650.jpg)

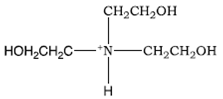


![T23040-1.0 - Triethanolamine, Free Base [2,2',2"-Nitrilothriethanol], 1 Liter T23040-1.0 - Triethanolamine, Free Base [2,2',2"-Nitrilothriethanol], 1 Liter](https://d2gdaxkudte5p.cloudfront.net/system/images/T23040-1.0_4.jpg)