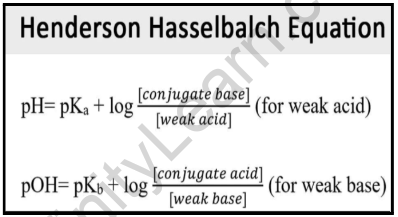
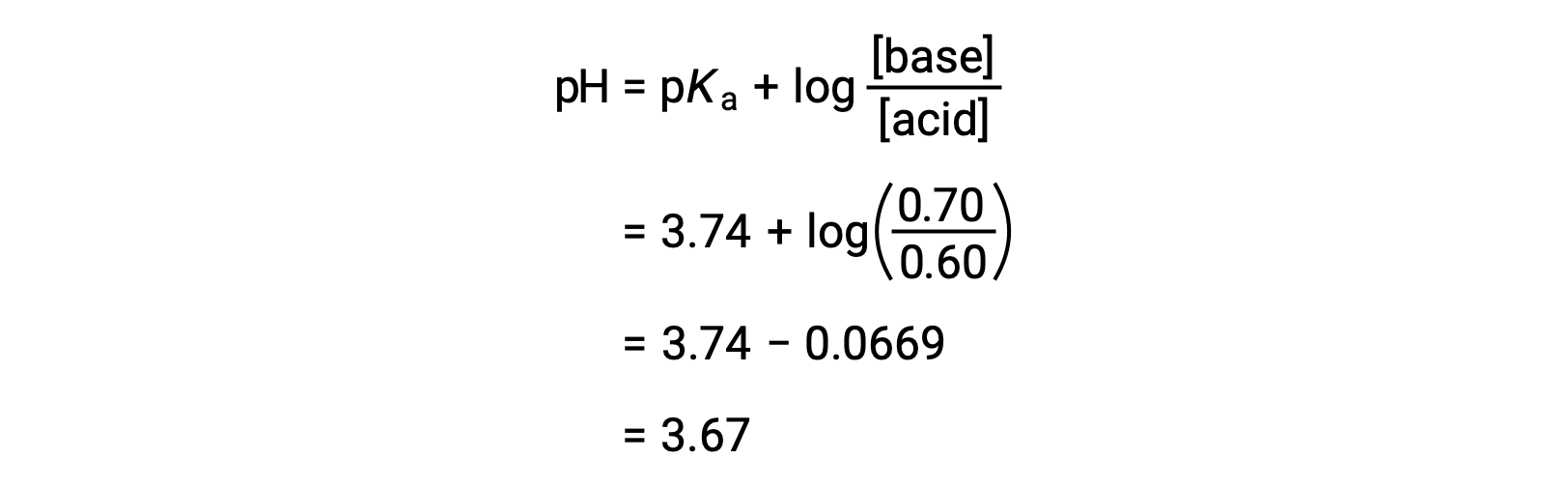![SOLVED: Henderson-Hasselbalch Equation (YOu must be able to derive this equation): [conjugate base] [A-] pH pKa + log OR pH = pKa + log [acid] [HA] 0. 1. What is the pH SOLVED: Henderson-Hasselbalch Equation (YOu must be able to derive this equation): [conjugate base] [A-] pH pKa + log OR pH = pKa + log [acid] [HA] 0. 1. What is the pH](https://cdn.numerade.com/ask_images/b0b5d45010ed4627b06a3a093e4b025c.jpg)
SOLVED: Henderson-Hasselbalch Equation (YOu must be able to derive this equation): [conjugate base] [A-] pH pKa + log OR pH = pKa + log [acid] [HA] 0. 1. What is the pH

BT_GS 1.9 Describe factors influencing the distribution of drugs (for example …. pH, pKa) …. | Primary LO of the Day


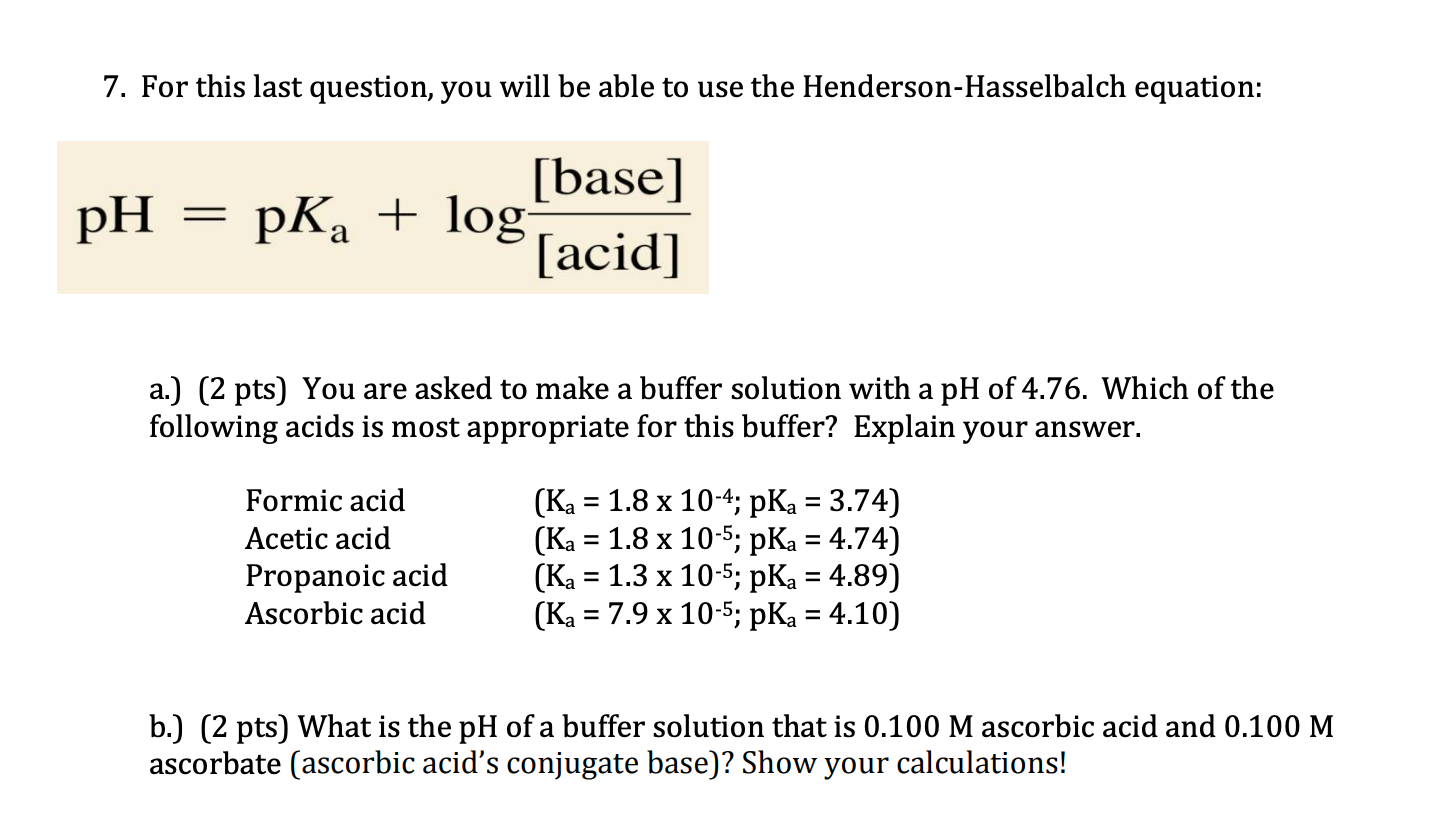
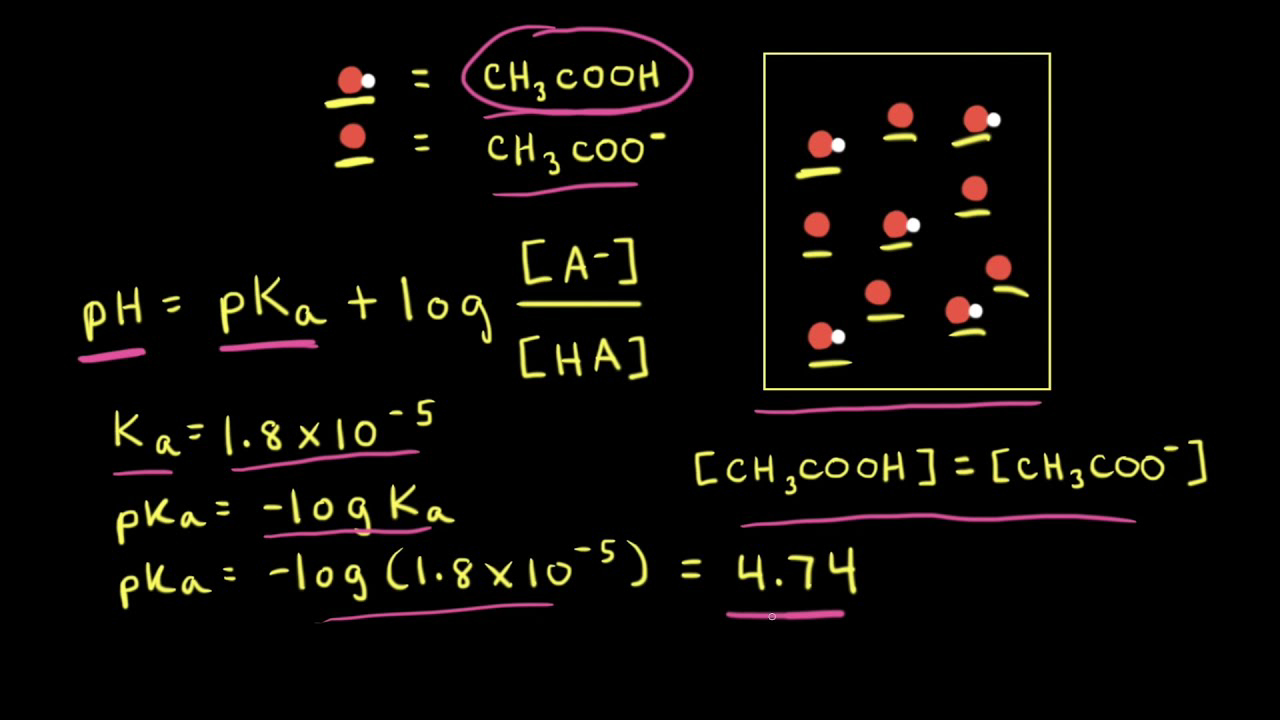
![Biochemistry | Henderson-Hasselbalch Equation Proof [pH=pKa] - YouTube Biochemistry | Henderson-Hasselbalch Equation Proof [pH=pKa] - YouTube](https://i.ytimg.com/vi/2jpB30LsT8g/maxresdefault.jpg)
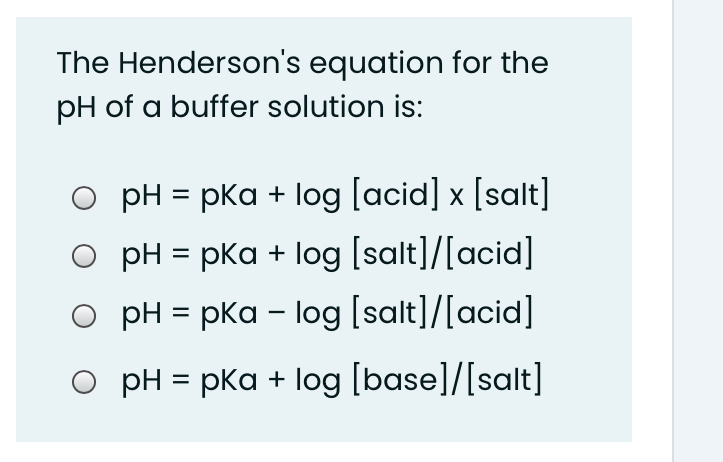
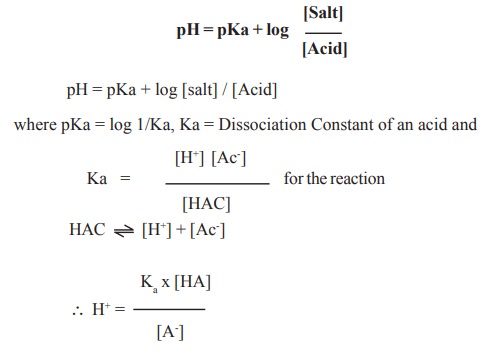
![Solved Important equations pH = pKa + log[salt]/[acid] | Chegg.com Solved Important equations pH = pKa + log[salt]/[acid] | Chegg.com](https://media.cheggcdn.com/media/14b/14b35f43-4468-42d2-a7e2-d924b3fd6eba/phpQPtBXO.png)
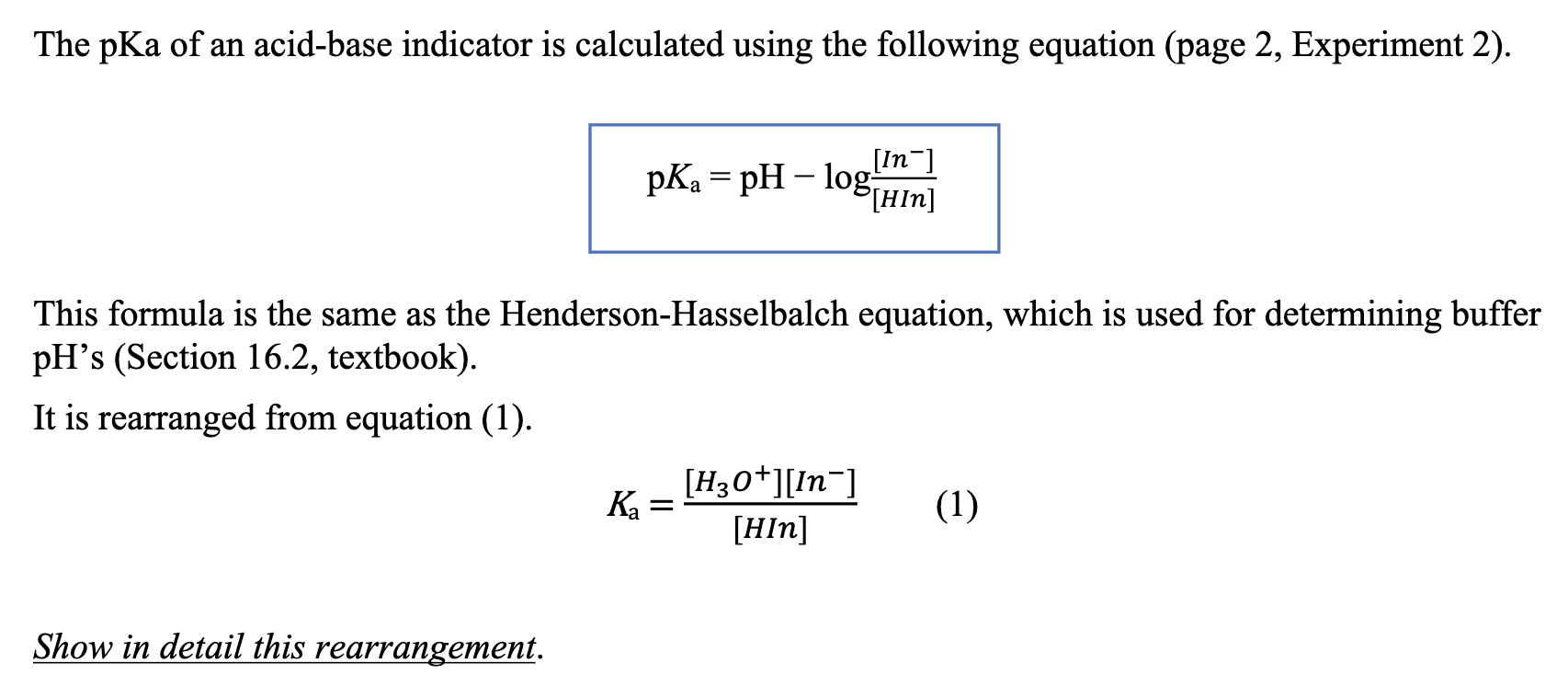


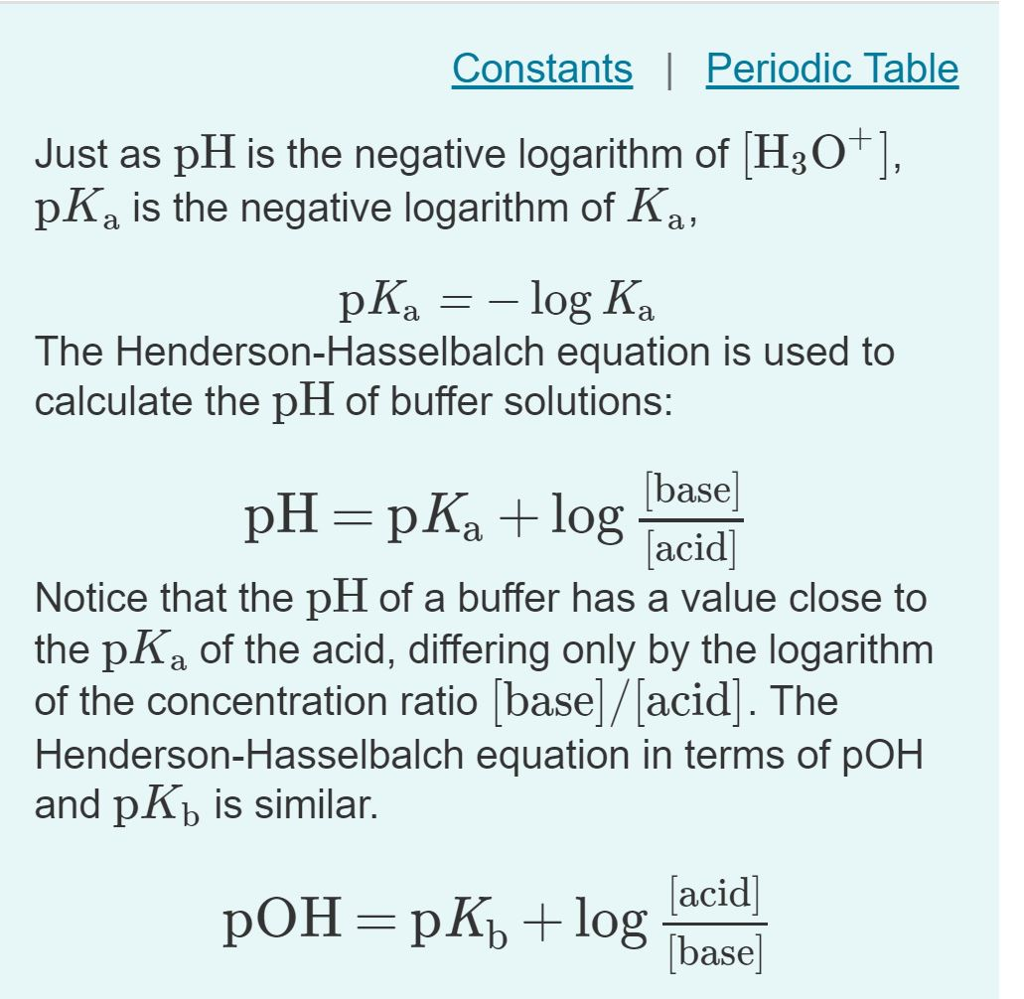
![SOLVED: [A-] log [HA] pKa pH d. [A- ] PH = PKa + log [HA] [A-] PH = pKa + [HA] SOLVED: [A-] log [HA] pKa pH d. [A- ] PH = PKa + log [HA] [A-] PH = pKa + [HA]](https://cdn.numerade.com/ask_images/959424b8320b49c898de609f834a314d.jpg)