ntCalculate the percentage ionization of 0.01M acetic acid in 0.1M HCL Ka of acetic acid is 1.8 10 5(10 to the power 5)n

Calculate the percentage ionization of 0.01 M acetic acid in 0.1 m HCl . Ka of acetic acid is 1.8 × 10^-5 .

Worked example: Finding the percent ionization of a weak acid | AP Chemistry | Khan Academy - YouTube
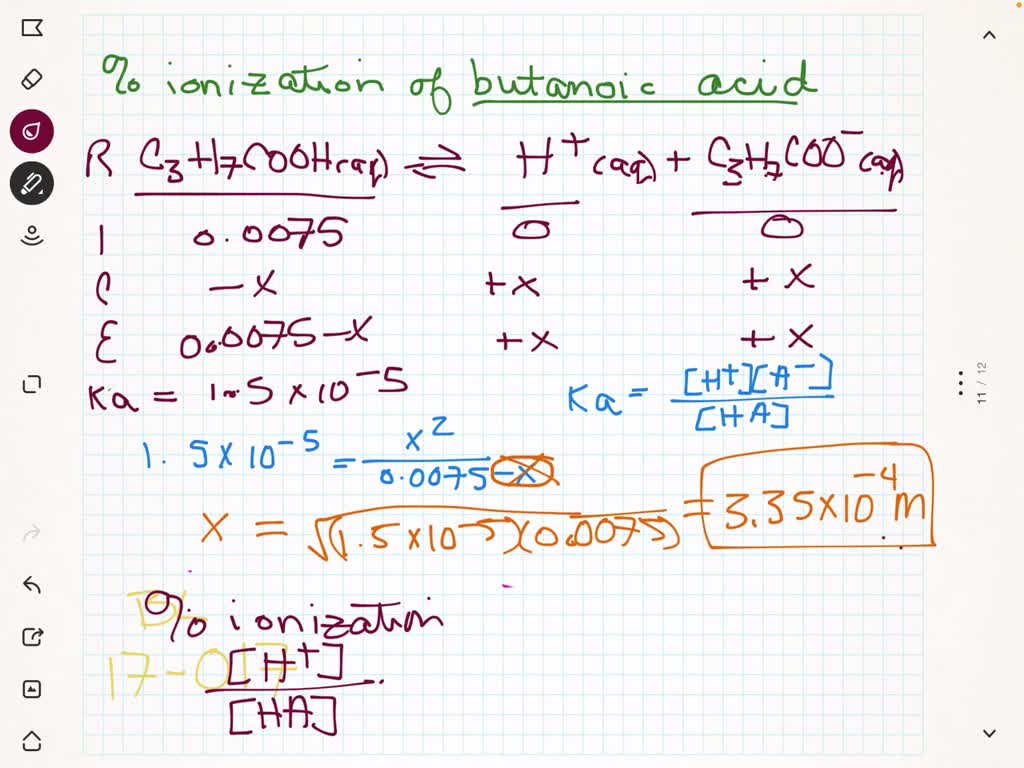
SOLVED:(a) Calculate the percent ionization of 0.0075 M butanoic acid (Ka=1.5 ×10^-5) . (b) Calculate the percent ionization of 0.0075 M butanoic acid in a solution containing 0.085 M sodium butanoate.
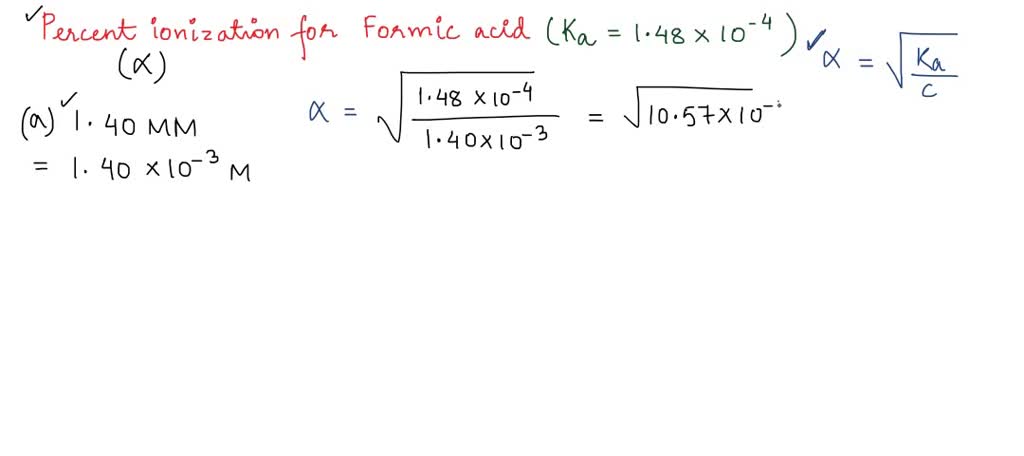
SOLVED: Calculate the percent ionization of formic acid solutions having the following concentrations. Ka = 1.8x10-4 Part B 0.460 M Express your answer using two significant figures. Express your answer using two
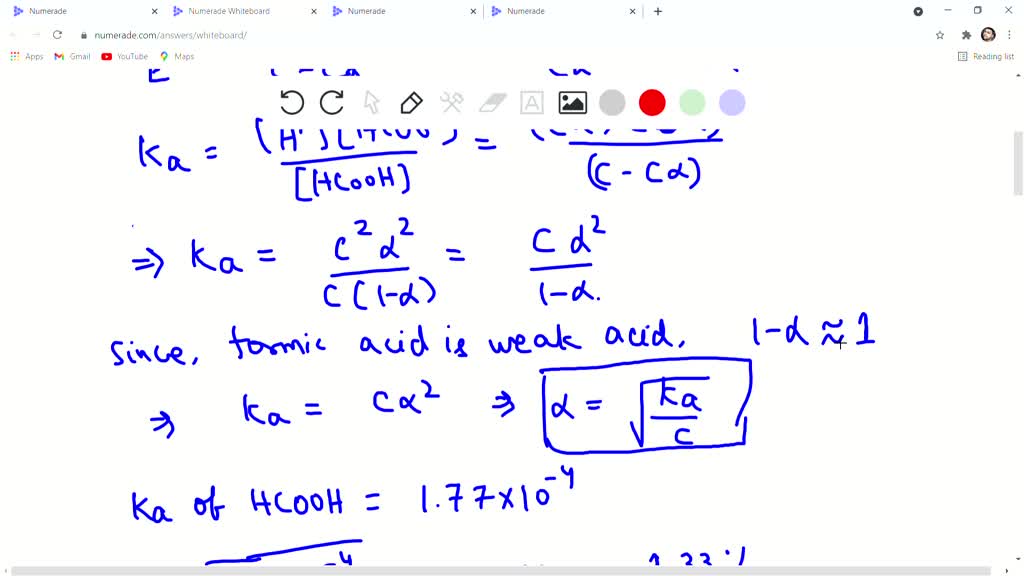
SOLVED: Calculate the percent ionization of a formic acid solution having the given concentrations. a. 1.00 M b. 0.500 M c. 0.100 M d. 0.0500 M