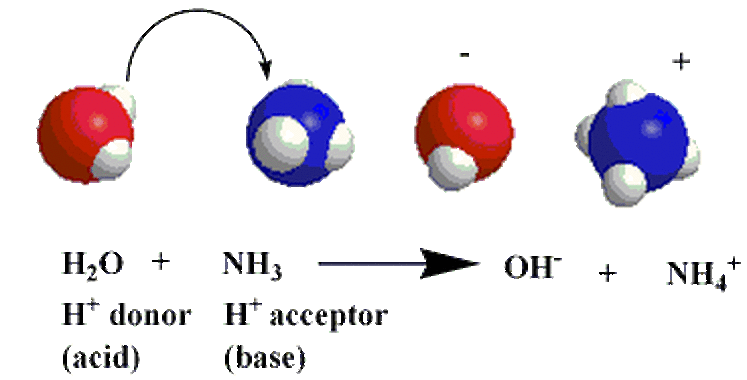
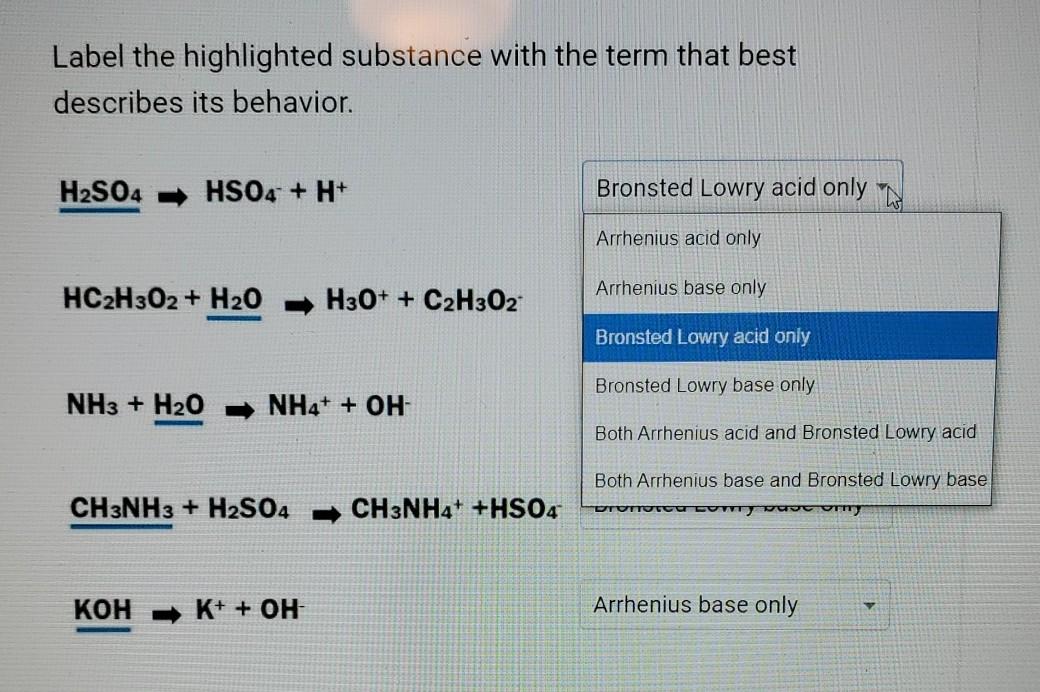
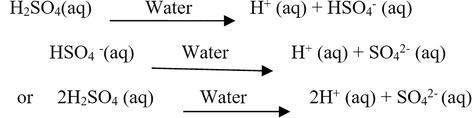
Arrhenius Base Overview & Examples | What is an Arrhenius Base? - Video & Lesson Transcript | Study.com
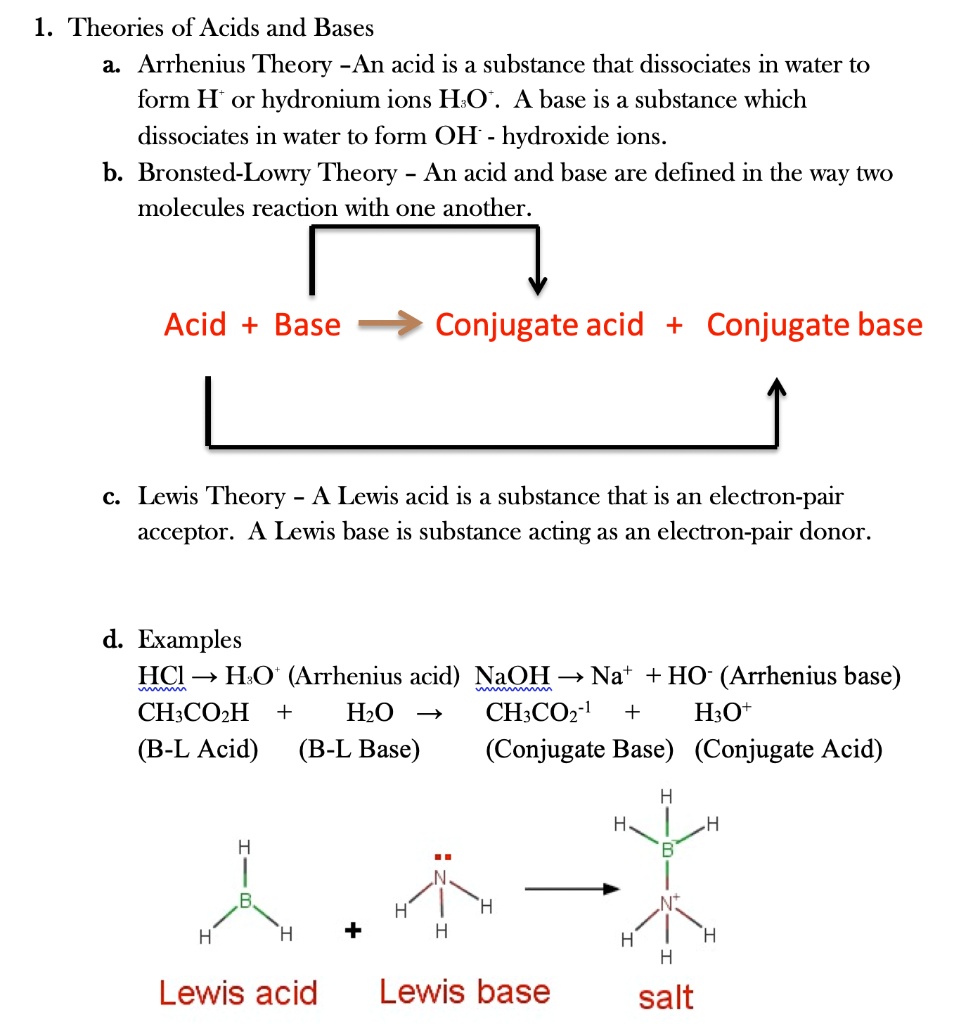
SOLVED: 1 Theories of Acids and Bases Arrhenius Theory -An acid is a substance that dissociates in water to form H or hydronium ions HO . A base is a substance which

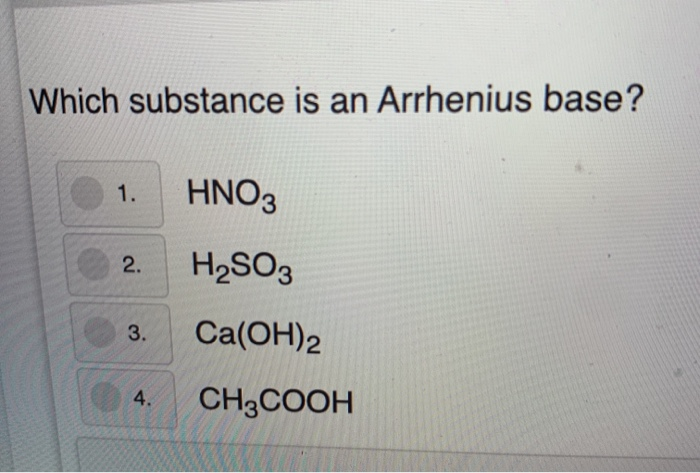
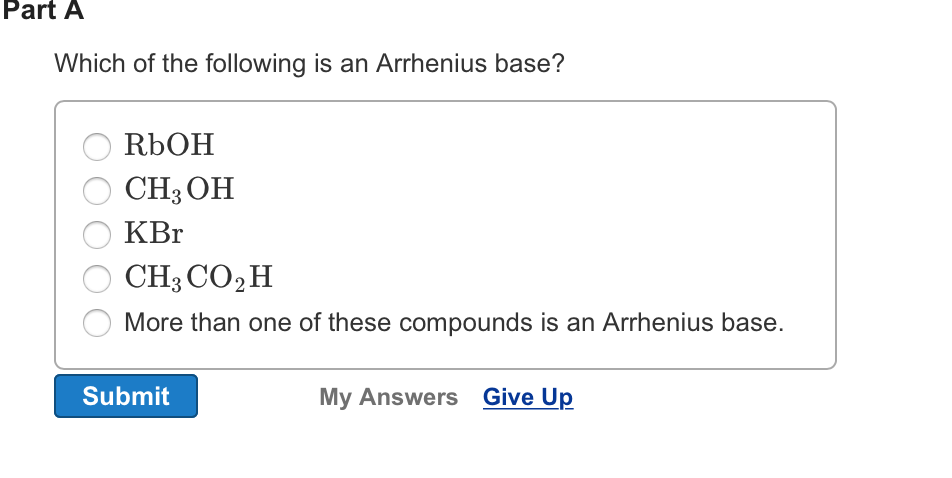
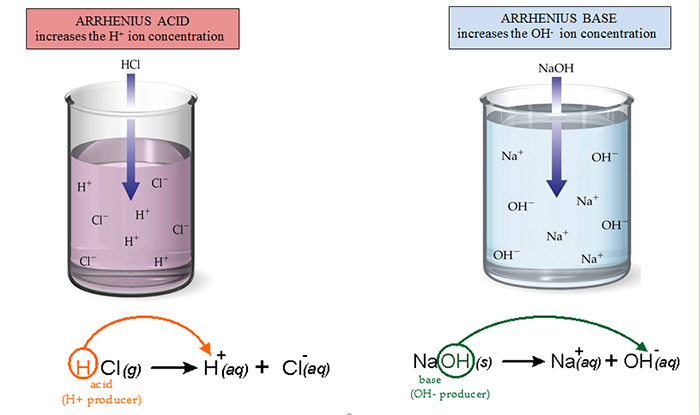
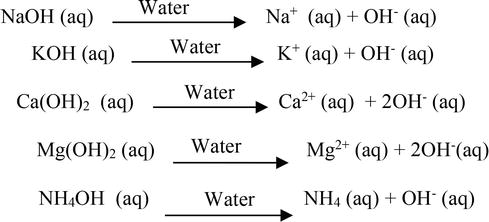

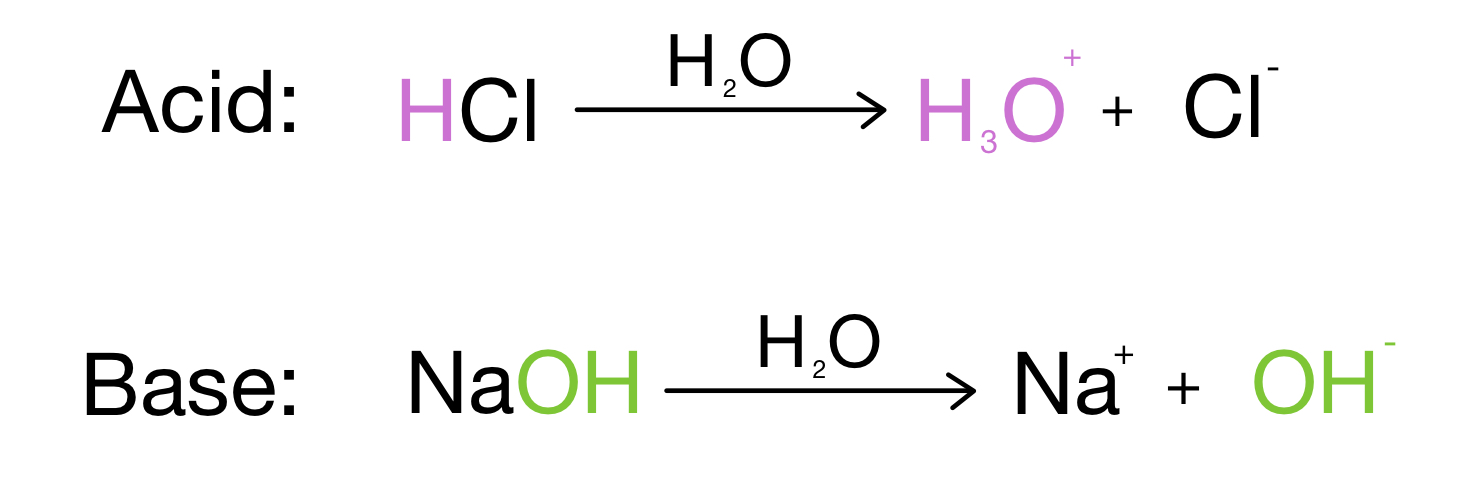



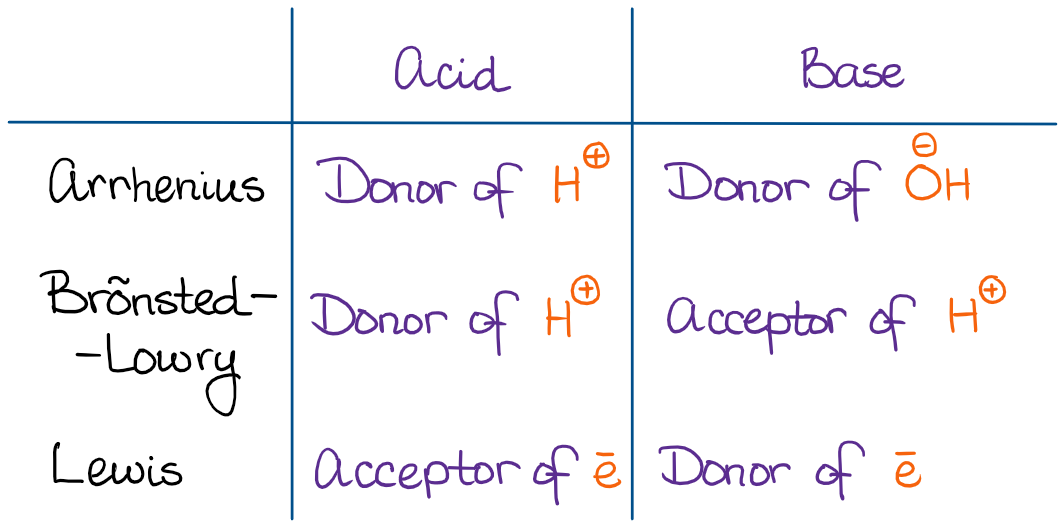




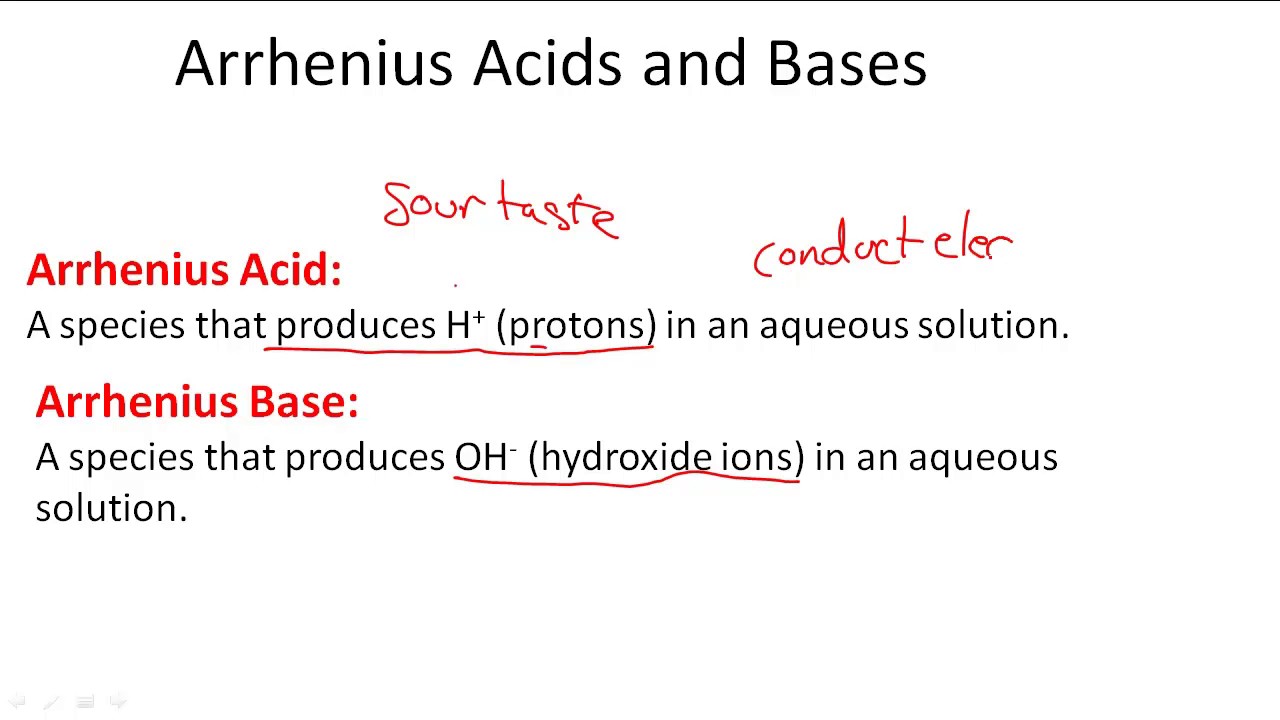
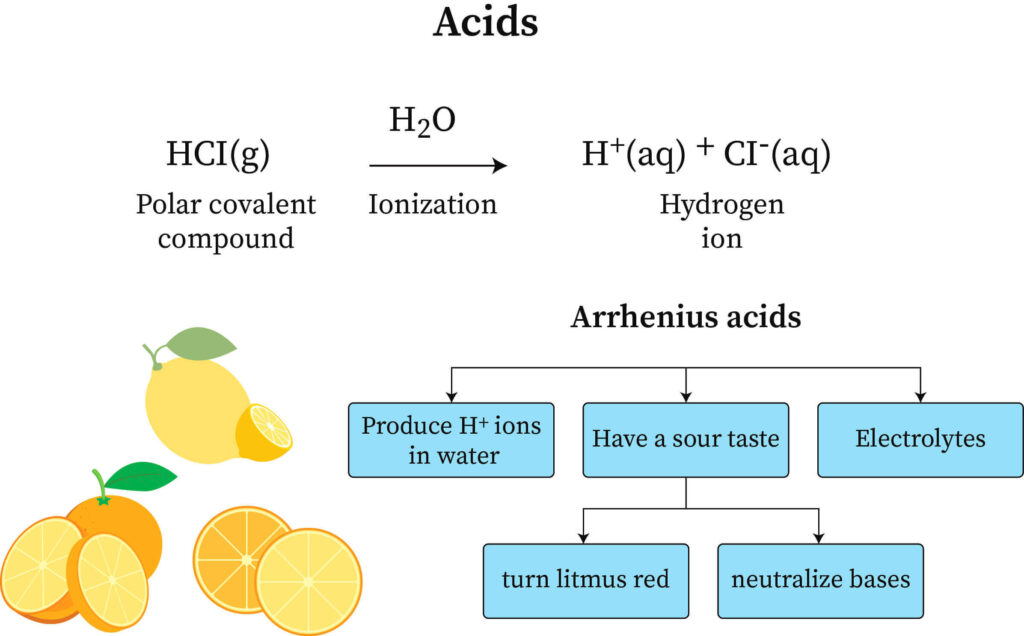
.PNG)